Characterizing Protein Structural Dynamics with Infrared Spectroscopy
Like a macroscopic machine, proteins have many moving parts. Their complete molecular-level description therefore must extend beyond the current static structure-function paradigm and include the potential population of multiple states (conformational heterogeneity) and their rates of interconversion (protein dynamics). Our group takes advantage of the very fast (picosecond) inherent timescale of infrared (IR) spectroscopy and the high spatial resolution afforded by the small size of IR chromophores to develop approaches for the rigorous characterization of localized environments anywhere in a protein and the measurement of dynamics on even the fastest timescales. We combine linear and advanced two-dimensional (2D) IR techniques with methods of biochemistry and chemical biology for placing vibrational probe groups at specific sites to map out the dynamics throughout proteins with atomic spatial resolution and femtosecond temporal resolution. This enables us to evaluate how conformational heterogeneity and dynamics contribute to biological function.
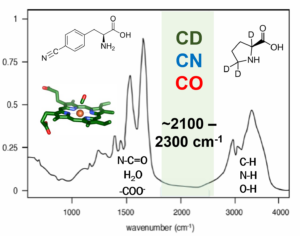
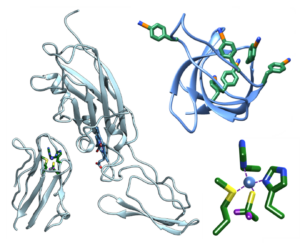
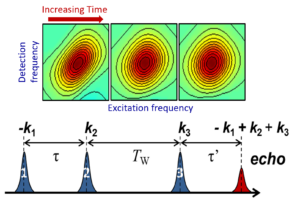
Vibrational Probes Biological Systems Spectroscopic Methods